 Plug in and turn on the hydrogen discharge lamp.
Hydrogen gas is excited by a current flowing through the gas. Look
at the light emitted by the excited gas through your spectral glasses.
You will see the line spectrum of hydrogen.
Plug in and turn on the hydrogen discharge lamp.
Hydrogen gas is excited by a current flowing through the gas. Look
at the light emitted by the excited gas through your spectral glasses.
You will see the line spectrum of hydrogen.In this studio session you will measure the wavelengths of the visible spectral lines in the Balmer series of hydrogen, inspect the spectral lines of helium, neon, and the output of a He-Ne laser, and use a simulation to build a laser.
Equipment needed:
Open a Microsoft Word document to keep a log of your procedures, results and discussions. This log will become your lab report. Address the points highlighted in blue. Answer all questions.
Experiment 1
In quantum mechanics confinement leads to energy quantization. The energy levels of the electron in a hydrogen atom are quantized. The allowed energies are
En = -13.6 eV/n2.
When an electron changes from one energy level to another, the energy of the atom must change as well. This energy can be supplied by a photon whose energy E = hf = hc/λ.
Since the energy levels are quantized, only certain photon wavelengths can be
absorbed. If a photon is absorbed, the electrons will be promoted to
higher energy levels and will then fall back down into the lowest energy state
(ground state) in a cascade of transitions. Each time the energy level of
the electron changes, a photon will be emitted and the energy (wavelength) of
the photon will be characteristic of the energy difference between the initial
and final energy levels of the atom in the transition. The energy of the
emitted photon is just the difference between the energy levels of the initial (ni)
and final (nf) states.
The set of spectral lines for a given final state nf are generally
close together. The lines for which nf = 2 are called the
Balmer series and many of these spectral lines are visible. You will be
measuring the wavelengths of the Balmer series lines.
The photon energies E = hf for the Balmer series lines are given by the formula
hf = -13.6 eV(1/ni2 - ½2) = 13.6 eV(1/4 - 1/ni2).
We may write hc/λ = 13.6 eV(1/4 - 1/ni2), or
1/λ = (13.6 eV/(hc))(1/4 - 1/ni2) = R(1/4 - 1/ni2).
The constant R is called the Rydberg constant. You will determine the Rydberg constant in this experiment.
 Plug in and turn on the hydrogen discharge lamp.
Hydrogen gas is excited by a current flowing through the gas. Look
at the light emitted by the excited gas through your spectral glasses.
You will see the line spectrum of hydrogen.
Plug in and turn on the hydrogen discharge lamp.
Hydrogen gas is excited by a current flowing through the gas. Look
at the light emitted by the excited gas through your spectral glasses.
You will see the line spectrum of hydrogen.
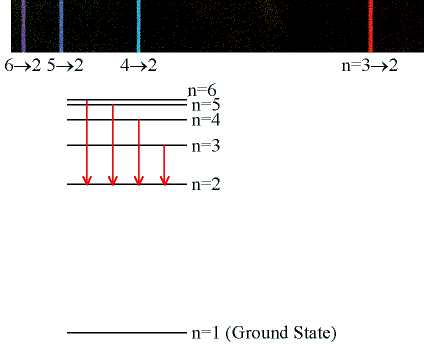
Enter each wavelength in units of nm into a spreadsheet.
| ni | color | λ (nm) | 1/λ (nm-1) | (1/4 - 1/ni2) |
|---|---|---|---|---|
| 6 | v | |||
| 5 | vb | |||
| 4 | bg | |||
| 3 | r |
Note: If the Spectrometry program is not available, you can use the Capstone program the find the wavelength of the spectral lines hydrogen spectrum by following these instructions.
Exploration 1
Lasers produce highly coherent light via stimulated emission. A pumping mechanism has to produce a population inversion and a cavity is needed to recycle the photons and increase the probability of stimulated emission. The class notes introduce you to the He-Ne laser.
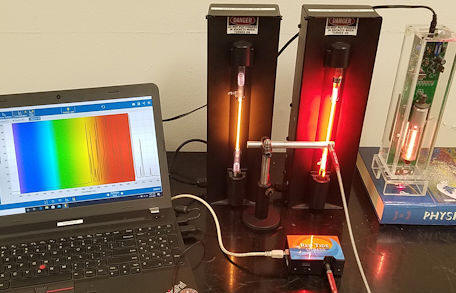
We have use the Red Tide spectrometer to measure 3 line spectra, the spectra emitted He and Ne discharge lamps and the spectrum emitted by a open-cavity He-Ne laser discharge tube. We have also recorded spectral line of the laser beam.
The spectrum you will analyze was measured using a Red Tide spectrometer.
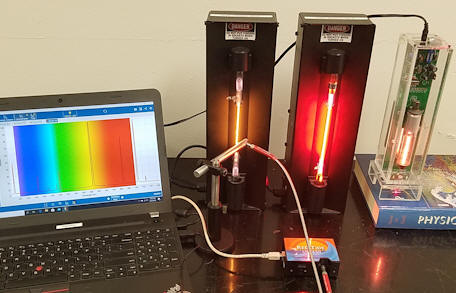 |
 |
| Spectrum of He discharge tube | Spectrum of Ne discharge tube |
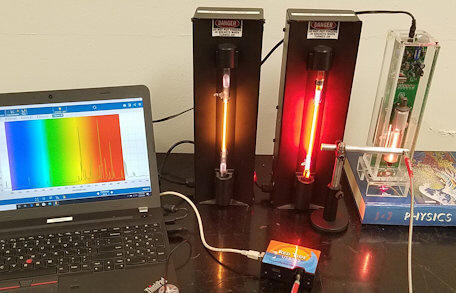 |
 |
| Spectrum of He-Ne laser cavity | Spectrum of laser beam shining into the fiber |
Download the spreadsheet He-Ne.xlsx and open it. Examine plots of the spectra.
Even a weak line in the spectrum of a gas can become a laser line if the conditions for stimulated emission are met.
Exploration 2
The class notes introduce you to a 4-level optical pumping scheme. In a 4-level scheme, the upper laser level may or may not be metastable.
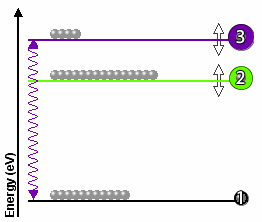 3-level optical pumping schemes are also possible, but
in a 3-level scheme the upper laser level has to be long-lived or
metastable.
3-level optical pumping schemes are also possible, but
in a 3-level scheme the upper laser level has to be long-lived or
metastable.
In the diagram on the right, a 3-level optical pumping
scheme is shown. Level 3 is the metastable upper laser level.
Use an on-line simulation from the University of
Colorado PhET group to build a laser.
Link to the simulation
http://phet.colorado.edu/en/simulations/lasers.
Open the One Atom Panel in the Laser Simulation and start exploring the two-level atom.
Explore the three-level atom.
Switch to the Multi-Atom Panel and build a laser.
You want to produce many identical photons.
Describe what you have to do to achieve this goal.
First try to establish a population inversion.
Now build a laser.
Convert your log into a lab report. See the grading scheme for all lab reports.
Name:
E-mail address:
Laboratory 11 Report
Save your Word document (your name_lab11.docx), go to Canvas, Assignments, Lab 11, and submit your document.